the InterStimTM Systems
Medtronic Bladder Control Therapy
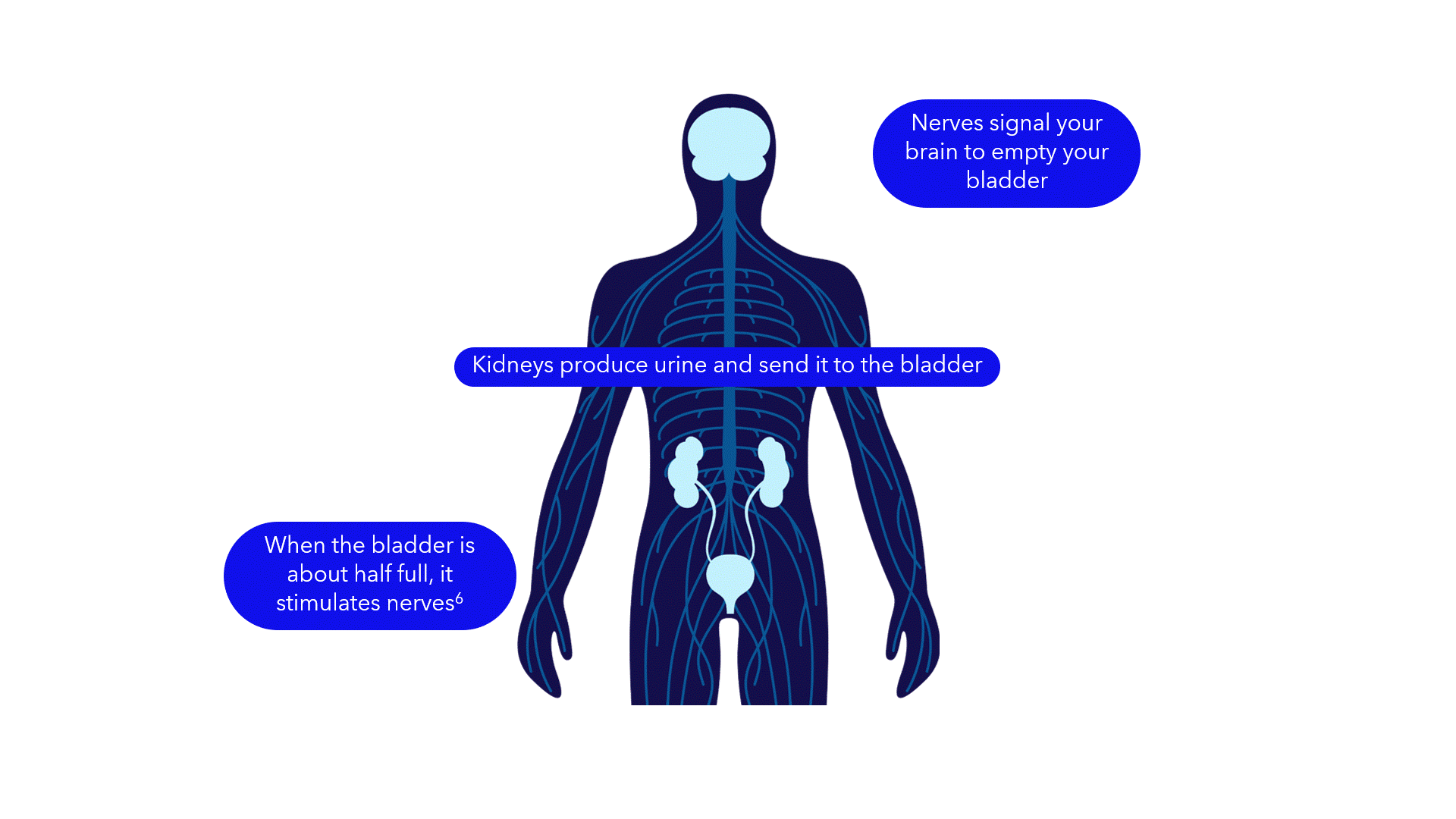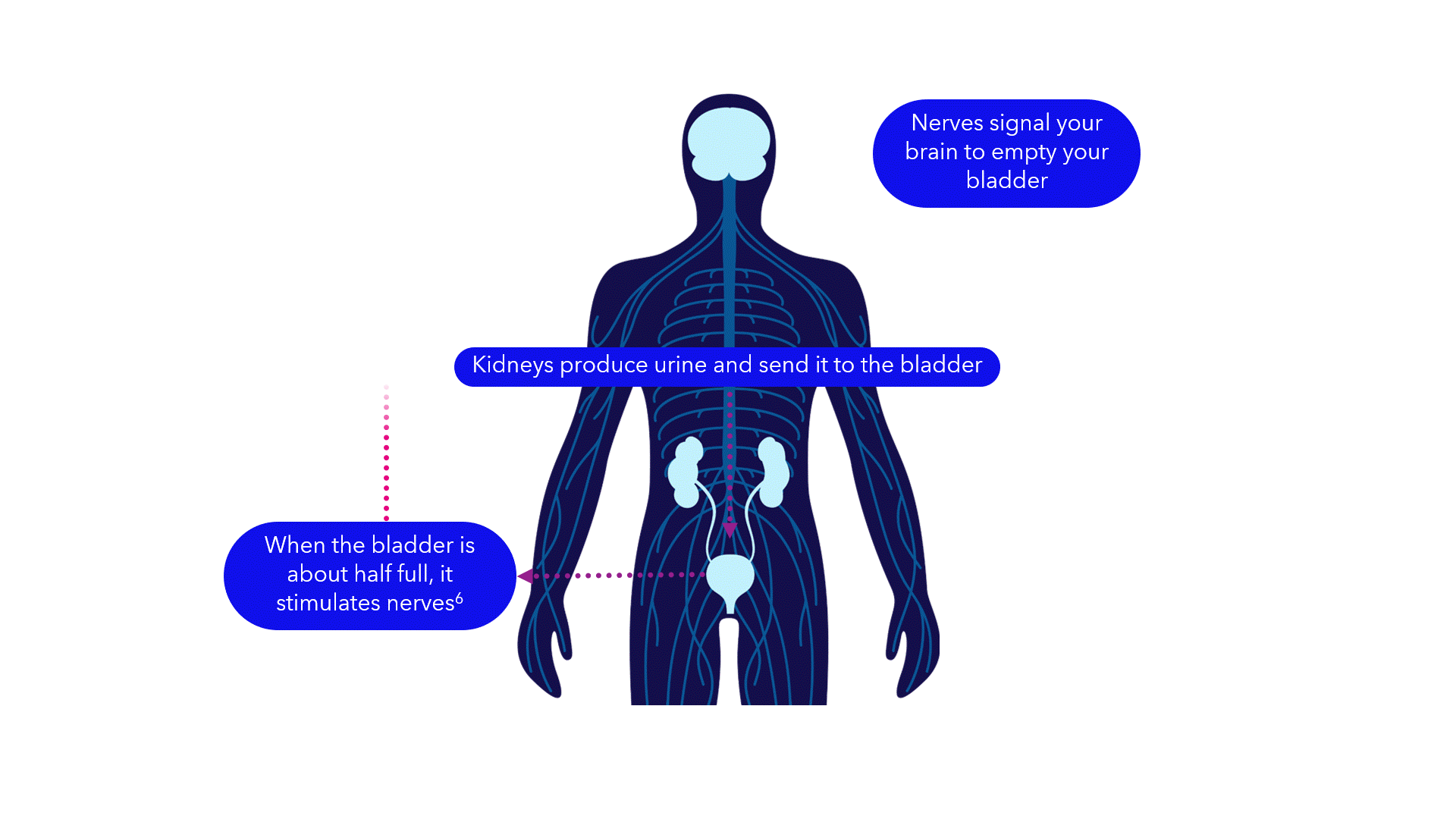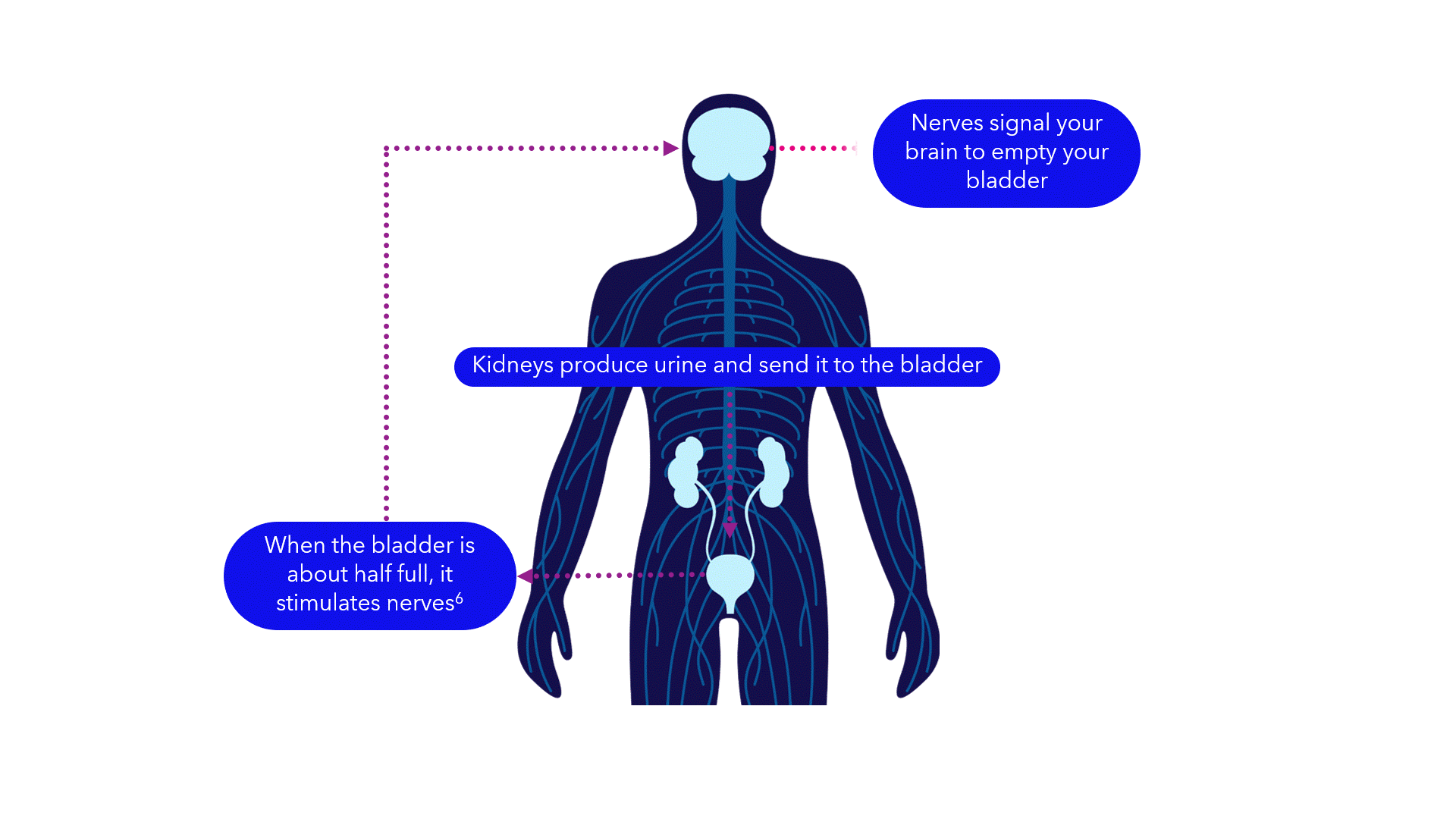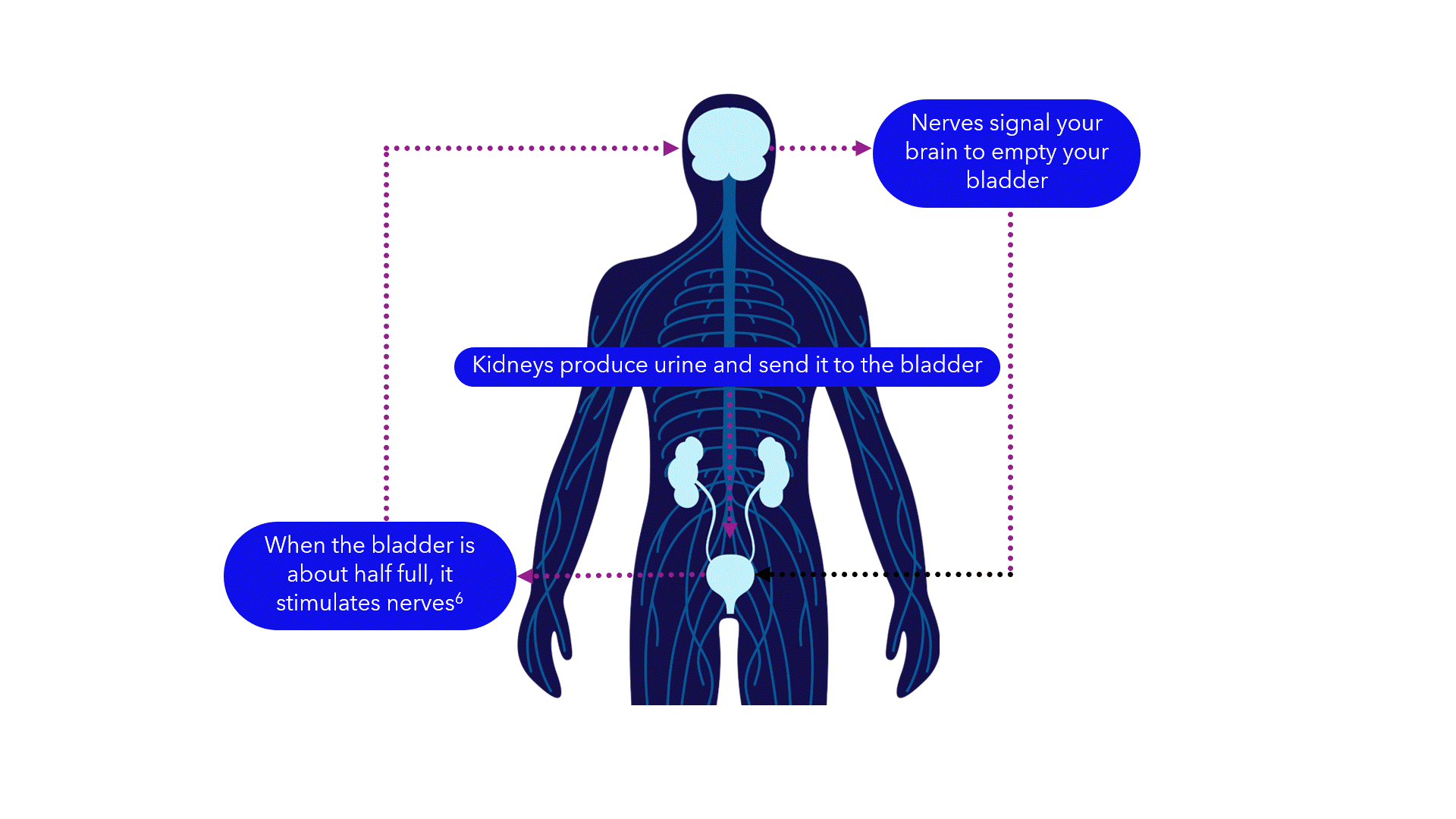
Evidence suggests that breakdowns in the bladder-brain communication pathway may be a root cause of OAB and non-obstructive urinary retention.1,2,3 That’s why conventional treatments may not produce the results you want – they don’t directly target this miscommunication. Unlike conventional treatments, the Medtronic InterStim systems gently stimulate the sacral nerves in the pelvic area that control the bladder.4,5 This may help restore* bladder-brain communication and reduce symptoms.
Get more control with the InterStim Systems6,7
In addition to risks related to surgery, complications can include pain at the implant sites, new pain, infection, lead (thin wire) movement/migration, device problems, undesirable changes in urinary or bowel function, and uncomfortable stimulation (sometimes described as a jolting or shocking feeling). Talk with your doctor about ways to minimize these risks.

Is InterStim Right for You?
You may be a good candidate for Medtronic Bladder Control Therapy delivered by the InterStim systems if:

frequently asked questions
InterStim™ Systems
Sources
†Numbers reflect completers analysis defined as patients with diary data at baseline and 12 months (n=220). Clinical success was 82% at 12 months using the modified completers analysis (subjects who either had a baseline and 12-month evaluation or withdrew early due to device-related reasons and are considered failures). Success defined as a 50% or greater reduction in your troublesome bladder symptoms.
‡Under certain conditions; see approved labeling for details. Patients with InterStim™ SureScan™ MRI Leads only.
1. Dasgupta R. Critchley HD, Dolan RJ, Fowler CJ. Changes in brain activity following sacral neuromodulation for urinary retention. J Urol. 2005; 174:2268-2272
2. Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005; 174:1862-1867.
3. Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27(6):466-474.
4. Kenefick NJ, Emmanuel A, Nicholls RJ. Effect of sacral nerve stimulation on autonomic nerve function. British Journal of Surgery. 2003;90:1256-1260.
5. Patton V, Wiklendt L, Arkwright JW, Lubowski DZ, Dinning PG. The effect of sacral nerve stimulation on distal colonic motility in patients with fecal incontinence. Br J Surg. 2013;100:959-968.
6. Siegel S, Noblett K, Mangel J, et al. Five-year follow-up results of a prospective, multicenter study of patients with overactive bladder treated with sacral neuromodulation. J Urol. 2018; 199(1), 229-236.
7. Medtronic InterStim Therapy Clinical Summary (2018).
8. Foster RT Sr, Anoia EJ, Webster GD, Amundsen CL. In patients undergoing neuromodulation for intractable urge incontinence a reduction in 24-hr pad weight after the initial test stimulation best predicts long-term patient satisfaction. Neurourol Urodyn. 2007; 26:213-217.
9. Siegel S, Noblett K, Mangel J, et al. Results of a prospective, randomized, multicenter study evaluating sacral neuromodulation with InterStim® Therapy compared to standard medical therapy at 6-months in subjects with mild symptoms of overactive bladder. Neurourol Urodyn. 2015; 34:224-230.
10. Foster RT Sr, Anoia EJ, Webster GD, Amundsen CL. In patients undergoing neuromodulation for intractable urge incontinence a reduction in 24-hr pad weight after the initial test stimulation best predicts long-term patient satisfaction. Neurourol Urodyn. 2007; 26:213-217.


